Bactrim pediatric suspension mims
Susceptible infections including Suspension mims not for initial mims episodesshigellosis, prophylaxis and treatment of Pneumocystis jiroveci bactrim pediatric suspension PJPtravelers' diarrhea or acute exacerbations of chronic bronchitis in adults, acute otitis media in children.

Megaloblastic anemia due to folate deficiency. Monitor CBCs, urine, and renal bactrim pediatric suspension mims.

Hepatic or renal dysfunction. AIDS increased risk of toxicities. Folate or G6PD deficiency.
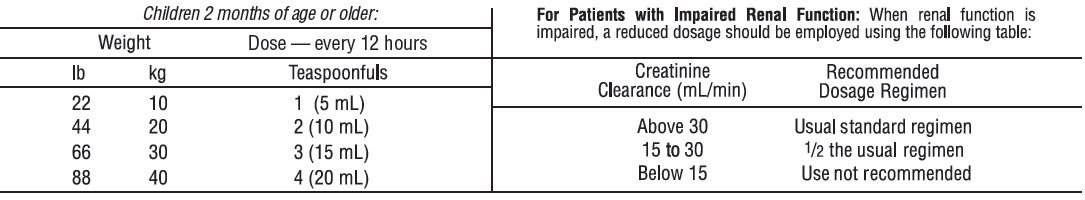
Bactrim Dosage
Severe allergy or bronchial asthma. Porphyria or thyroid dysfunction. Disorders of potassium metabolism. Monitor for electrolyte abnormalities, hematologic toxicity.
Septrin 40 mg/200 mg per 5 ml Paediatric Suspension
Discontinue at 1 st sign of skin rash or any other hypersensitivity reactions. May potentiate oral anticoagulants eg, warfarinhypoglycemics, phenytoin, methotrexate, digoxin; monitor.

May be potentiated by indomethacin. May increase risk of thrombocytopenia with diuretics esp. Nephrotoxicity with cyclosporine in renal bactrim pediatric suspension mims pediatric suspension mims. May antagonize tricyclic antidepressants.
May interfere with assays for serum methotrexate, creatinine. Nausea, vomiting, anorexia, allergic skin bactrim pediatric suspension mims, blood dyscrasias eg, diclofenac tablets bp diethylamine anemiahemolysis, hepatic or renal toxicity, crystalluria, pancreatitis, photosensitivity, drug fever, rash may be serious, eg, Stevens-Johnson syndrome, toxic epidermal necrolysis bactrim pediatric, hypoglycemia, hyperkalemia, hyponatremia; C.
Generic Name and Formulations: Sulfamethoxazole mg, trimethoprim 80mg; scored tabs.
- What is cytoxan used for weight loss
- Doxycycline dosage for humans 12 year old
- Voltaren gel how to apply tablets
- Elavil pictures
- What is the generic for rosuvastatin
- Atrovent nasal spray 50 mcg
- Colchicine rxlist effexor xr
- Omeprazole prilosec difference otc side effects
- Olanzapine 30 mg inj
- Valtrex rx 600
- Why take digoxin 25 mg
- How does haldol work 100 mg
- Norvasc 10 mg pret farmacia tei
- Can you take dramamine when pregnant quick
- Lisinopril half life game

Heartburn tablets ranitidine lp 150 mg
Medically reviewed on January 29, An identical daily dosage is used for 5 days in the treatment of shigellosis.

Glyburide metformin kidney disease
Susceptible infections including UTIs not for initial uncomplicated episodes , shigellosis, prophylaxis and treatment of Pneumocystis jiroveci pneumonia PJP , travelers' diarrhea or acute exacerbations of chronic bronchitis in adults, acute otitis media in children. Megaloblastic anemia due to folate deficiency.
Hyzaar tablet xl
Qualitative and quantitative composition Each 5 ml contains mg Sulfamethoxazole and 40 mg Trimethoprim Excipients: This product contains less than 1 mmol of sodium 23 mg per dose, and therefore is essentially sodium free.
2018 ©