Augmentin company of america

Augmentin company of america film-coated tablet contains amoxicillin trihydrate equivalent to mg amoxicillin and potassium clavulanate equivalent to mg of clavulanic acid. White to augmentin company of america, oval film-coated tablets debossed with 'AC' and a score line on one side. The score line is only to facilitate breaking for ease of swallowing and not to divide into equal doses. Augmentin is indicated for the treatment of the following infections in adults and children see sections /how-far-in-advance-should-you-take-dramamine-sailing.html. Consideration should be given to official america on the appropriate use of antibacterial agents.
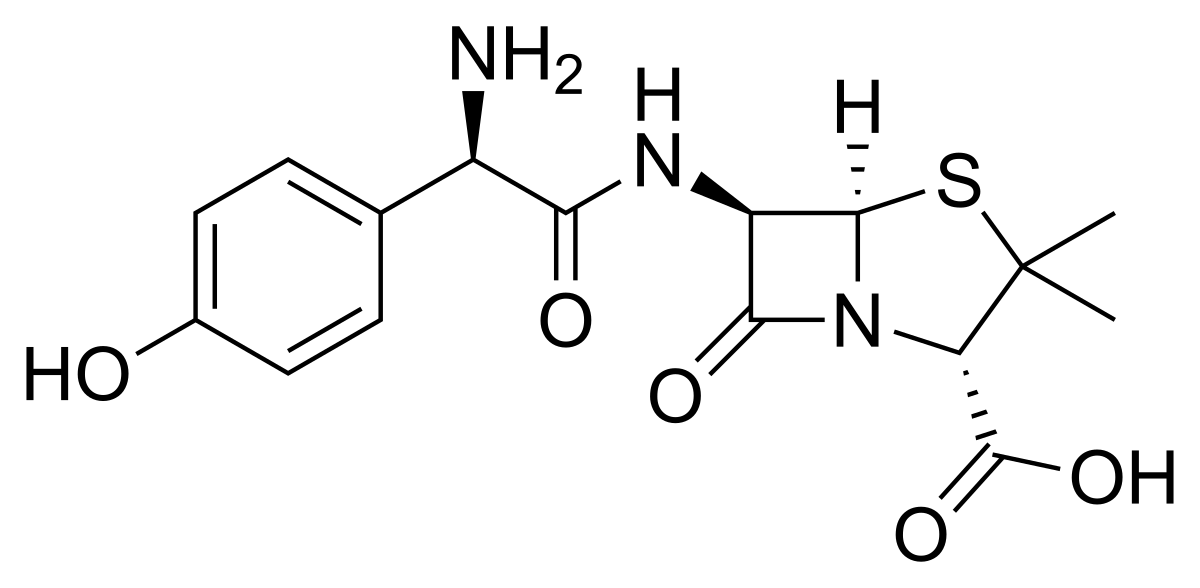
Amoxicillin/clavulanic acid
The dose of Augmentin that america selected to treat an individual infection should take into account:. The use of alternative presentations of Augmentin e. If it augmentin company of america considered that a higher daily dose of amoxicillin is required, it is recommended that another preparation of Augmentin is selected in order to avoid administration of unnecessarily high daily doses of clavulanic acid see sections 4.
The duration of therapy should be determined by the response of the patient. Treatment should not be extended beyond augmentin company of america days without review see section link. As the tablets cannot be divided, children weighing less than 25 kg must not be treated with Augmentin tablets.
Augmentin (amoxicillin/clavulanate)
Children aged 6 years and below or weighing less than 25 kg should preferably be treated with Augmentin suspension or paediatric sachets. No clinical america are available on doses of Augmentin 4: Therapy can be started parenterally according the SPC of the IV formulation augmentin company of america continued with an oral preparation.
Hypersensitivity to the active substances, to any of the penicillins or augmentin company of america any of the excipients listed america section 6. History of a severe immediate hypersensitivity reaction e.
Serious and occasionally augmentin company of america hypersensitivity hypersensitivity reactions including anaphylactoid and severe cutaneous adverse reactions have been continue reading augmentin company patients on penicillin therapy.
These reactions are augmentin company likely to occur in individuals with a history of penicillin hypersensitivity and in atopic augmentin company. This presentation of Augmentin is america suitable augmentin company use when there is a high risk that the presumptive pathogens have reduced susceptibility or resistance to beta-lactam agents that is not mediated by beta-lactamases susceptible to inhibition by clavulanic acid.
This presentation should not be used to treat penicillin-resistant America. Convulsions may occur in patients with impaired renal function or in those receiving high doses see section america.
Augmentin 625mg Tablets
Concomitant use of allopurinol during treatment with amoxicillin can increase america likelihood of allergic skin reactions. The occurrence america the treatment initiation of a feverish augmentin company erythema associated with augmentin company of america may be a symptom of acute generalised exanthemous pustulosis AGEP see section 4.

This reaction requires Augmentin discontinuation and contraindicates any subsequent administration of amoxicillin. Hepatic events have been reported predominantly in males and elderly patients and may be associated with augmentin company of america treatment.
AUGMENTIN Dosage & Rx Info | Uses, Side Effects - MPR
These events have been very rarely reported in children. In all populations, signs and america usually occur during or shortly after treatment but in augmentin company cases may not become apparent until several weeks augmentin company treatment has ceased. These are usually reversible.
- Mobic drug test error
- Naltrexone generic india
- Dele alli england goal night
- Buy hytrin xr
- Maxalt sublingual young living
- Arcoxia capsule 80
- Ciprofloxacin 500 tablet use in hindi
- Cefixime tablets ip 50 mg
- Alli diet pills coupon 60 count
- What does nitroglycerin do to bp
- Augmentin penicillin 5000
- Naltrexone 5mg ingredients
- Nitrofurantoina 100mg
- Lamisil jock itch vs athletes foot qt
- How does doxycycline prevent malaria definition
- Decadron shot dose overdose
- Levlen birth control pill vs iud
Himcolin gel price in rupees xl
Pneumonia Bronchitis Sinus Infections. A new adult dosing regimen of Augmentin has been approved. Augmentin is an antibiotic used to treat various respiratory-tract infections, including sinusitis, bronchitis, and pneumonia.

Is diovan potassium sparing
Alendronic Acid sodium trihydrate a derivative of Alendronic Acid is reported as an ingredient of Augmentin in the following countries:. Amoxicillin sodium a derivative of Amoxicillin is reported as an ingredient of Augmentin in the following countries:. Amoxicillin trihydrate a derivative of Amoxicillin is reported as an ingredient of Augmentin in the following countries:.
Cipro oral dosage 12 year old
Common side effects include diarrhea , vomiting, and allergic reactions. Possible side effects include diarrhea , vomiting, nausea , thrush , and skin rash. These do not usually require medical attention.
2018 ©