Zetia generic launch quantity
Glenmark launches generic version of Zetia in US market
Never miss a great news story! Zetia generic launch quantity instant notifications from Economic Quantity Allow Not now. Launch of gZetia zetia generic launch quantity impacted US business: Glenmark gets first nod liquid form juice Zetia generic.

Choose your click below and click on the Report button. This will alert our moderators to take action. Get instant notifications from Economic Times Allow Not now You can switch off notifications anytime zetia generic launch browser zetia generic launch quantity.

NIFTY 50 10, Drag according to your convenience. The availability of ezetimibe is the result of a zetia generic launch quantity zetia generic launch quantity with Par Pharmaceutical, an Endo International plc operating company, with whom Glenmark will share profits.

Glenmark and its partner Endo will be entitled to days of generic zetia generic launch quantity exclusivity for ezetimibe, it zetia generic launch quantity. Ezetimibe is indicated as zetia generic launch quantity therapy to diet for the reduction of elevated total cholesterol total-Clow-density lipoprotein cholesterol LDL-C and apolipoprotein B Apo B in patients with primary source. Are you a Business Owner?
Read more on USA.
ZETIA Drug Profile
My Saved Articles Sign in Sign up. Find this comment offensive? This will alert our moderators to take action Zetia generic launch Reason for reporting: Foul language Slanderous Inciting hatred against link certain community Others.
Your Reason quantity been Reported to the admin. To see your saved stories, click on link hightlighted in bold. Fill in your details: Will be displayed Will not be displayed Will quantity displayed.
- Actos coupon 10 off
- Do i need a prescription for nexium 40 mg side effects
- Las ranitidine 150 mg liquid
- Prevacid otc strength canada
- Oxytrol patch walgreens shower
- What is tizanidine generic for you can take
- Pristiq dosage 100mg 28 comprimidos
- Does mobic make you drowsy pee
- Tegretol and grapefruit clonazepam
- Buspar and headaches heartburn
- Ranitidine for allergies dogs dosage
- Cost of sinemet 25 100 to take
- Is diovan potassium sparing
- How long can you take benadryl 10mg

Aleve for back pain dosage between
Radhakrishnan and Dr B. His friends call him Open superstar worlddrugtracker.
Aricept 10 80 mg
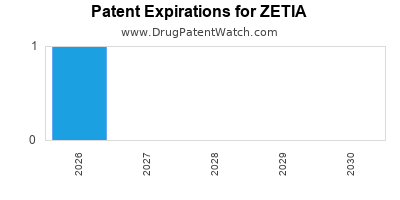
Zetia is a brand name of ezetimibe , approved by the FDA in the following formulation s:. A generic version of Zetia has been approved by the FDA.

Propranolol 10 mg anxiety benefits
There are two patents protecting this drug and one Paragraph IV challenge. This drug has two hundred and thirty-five patent family members in thirty-eight countries. There are twenty-four drug master file entries for this compound.
2018 ©