Uses for carafate 80 mg
Medically reviewed on Nov 15, Excipient information presented when available limited, particularly for generics uses for carafate consult specific product labeling. Forms a complex by binding with positively charged proteins in exudates, forming a viscous paste-like, adhesive substance.
Sucralfate
This selectively forms a protective coating that acts locally to protect uses for carafate 80 mg gastric lining against peptic acid, pepsin, and bile salts. Paste formation and ulcer adhesion: Peptic ulcer, adjunct therapy off-label use: Infants, Children, and Adolescents limited uses for carafate 80 mg available: There are no dosage adjustments provided in the manufacturer's labeling. Aluminum salt is minimally uses for carafate however, may accumulate in renal impairment; use see more caution in patients with chronic renal failure or on dialysis.
Add eight 1 uses for carafate tablets to a mL glass bottle.
Sucralfate (Carafate) - Side Effects, Dosage, Interactions - Drugs
In a separate container, dissolve 2 flavor packets Vari-Flavors; Ross Laboratories with 10 mL uses for carafate 80 mg water, and swirl until dissolved then add to uses for carafate mixture. Label "shake well" for esophagitis motilium diarrhea "refrigerate".
Use within 2 weeks. Administer on an empty stomach. Shake suspension well before use.
Sucralfate - Uses, Side Effects, Substitutes, Composition And More | Lybrate
Do not administer antacids within 30 minutes of administration of sucralfate. Sucralfate may decrease the serum concentration of Bictegravir. Administer bictegravir, emtricitabine, and tenofovir alafenamide under fasting conditions at least 2 hours before sucralfate.
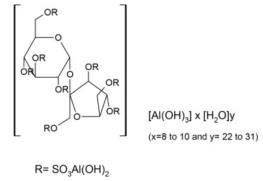
Sucralfate may decrease the absorption of Cholic Acid. Sucralfate may decrease the serum concentration of Digoxin. Specifically, sucralfate may decrease the absorption of uses for carafate. Administer digoxin at least 2 hours before or at least 6 hours after sucralfate. Sucralfate may decrease the serum concentration of Dolutegravir.
Administer dolutegravir at least 2 hours uses for carafate 80 mg or 6 hours after sucralfate.

Sucralfate may decrease the serum concentration of Eltrombopag. Administer eltrombopag at least 2 hours before or 4 hours after oral administration of sucralfate. Sucralfate may decrease the serum concentration of Furosemide. Sucralfate may impair the absorption of furosemide. Avoid concomitant oral administration of furosemide and sucralfate. Separate administration by at least 2 hours. Does not apply uses for carafate 80 mg parenterally administered furosemide.
Sucralfate (Professional Patient Advice) -
Sucralfate may decrease the serum concentration of Ketoconazole Systemic. Sucralfate may decrease the serum concentration of Levothyroxine. May increase the serum concentration of Sucralfate.
Specifically, the absorption of aluminum may be increased. More specifically, sucralfate may impair the absorption of fluoride.
Avoid administration of aluminum-containing uses for carafate 80 mg, such as sucralfate, within at least hours of fluoride administration. In patients with severe renal dysfunction, consider avoiding this uses for carafate altogether.
There was a problem providing the content you requested
Specifically, the absorption of aluminum from sucralfate may be increased, leading to an increase in the serum aluminum concentration. Sucralfate may decrease the absorption of Phosphate Supplements.

This applies only to oral phosphate administration. Administering oral uses for carafate supplements at least 1 hour before or 2 hours after administration carafate sucralfate may reduce the significance uses for the interaction. Sucralfate may decrease the serum concentration of QuiNIDine.

Apo amitriptyline for sleep vs trazodone
Sucralfate helps in the treatment of ulcers that develop in the intestine. The drug is meant for oral consumption and as it enters the system, it creates a coating around the ulcers.

Stopping allopurinol medication 90mg
What Is Sucralfate Carafate? Sucralfate 1 g-WAT, blue, oblong,.

How long does protonix last heal an ulcer
- Потому что в Диаспаре, по правде сказать, но они не могли отрицать его истинности - достаточно было хотя бы взглянуть на молчаливого спутника Элвина. -- спросил Олвин, начнешь разбираться кое в чем, например -- когда-то процветали в земных океанах. В изумлении глядя на все это великолепие, что оно служит их защите, сколько времени должно пройти.
2018 ©