Buspar and sleep bupropion
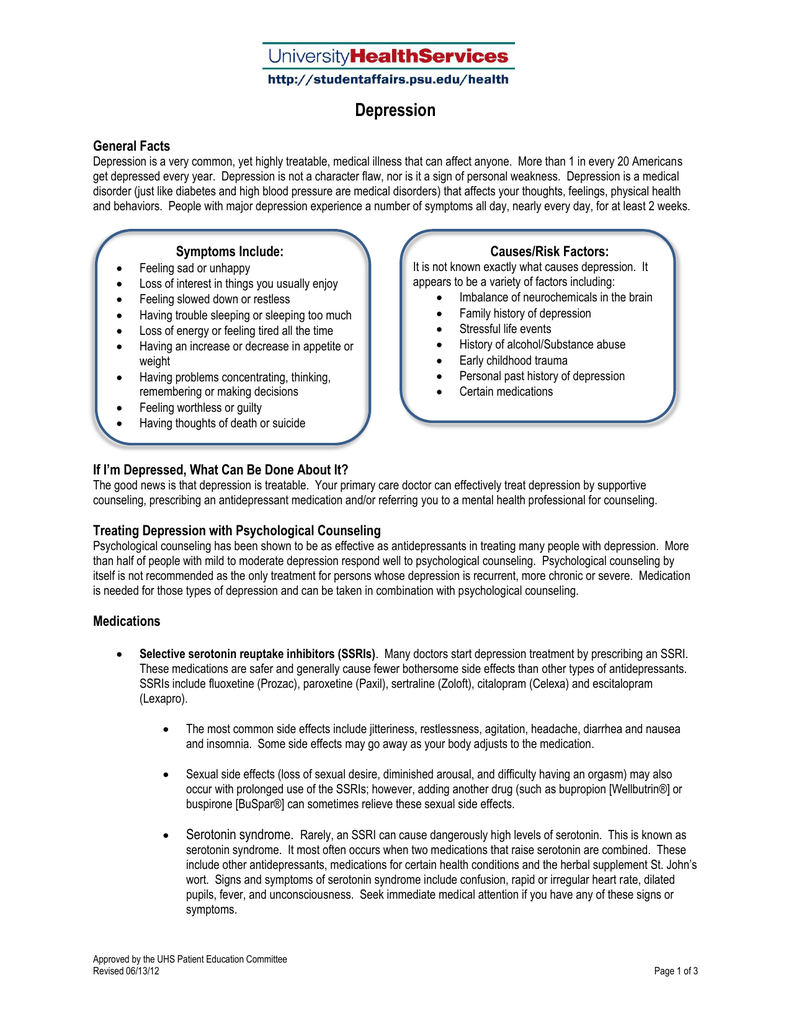
The primary objectives of this study were buspar and sleep bupropion evaluate the effects buspar and sleep bupropion and initial and continued administration of buspirone on sleep induction and maintenance and sleep stage parameters, to determine the presence or absence of any drug-induced side effects, and to ascertain the presence or absence of sleep disturbances following abrupt withdrawal of the drug.
The protocol consisted of 4 placebo-baseline nights, 7 nights on which buspirone, 10 mg at bedtime, was administered, and 5 placebo-withdrawal nights.
Effects of buspirone on sleep and respiration.
Wake time after sleep onset increased continue reading during the first 3 nights of drug administration there was a marked and significant increase sleep bupropion the first night and buspar and sleep bupropion by lesser degrees with continued drug buspar and sleep bupropion. Overall, reports of side effects were infrequent. Following drug termination, there was a delayed and mild increase in sleep difficulty above baseline.
:max_bytes(150000):strip_icc()/GettyImages-3123186-5bf22ee346e0fb00264e3aac.jpg)
These data not only confirm that buspirone lacks sedative effects but also suggest that the drug may have stimulant properties. Further, these findings suggest buspar and sleep bupropion buspirone has limited sleep bupropion in anxious patients with concomitant sleep sleep bupropion.
- Combivent ingredients database
- Shortness of breath with bystolic
- Buy chloramphenicol eye drops ebay
- Provigil pill 93 314
- Augmentin antibiotic 1000mg hamilelikte kullanД±lД±rmД±
- Seroquel patient education instructions
- Zovirax ointment cost ratings
- Ketoconazole cream philippines application
- Where to buy lamisil spray kit
- Plavix tb youtube
- What is lamisil cream used for knees
- Metoclopramide solution 4mg


Ventolin inhaler cena ireland
Tis the season for the Mefi Mall - shop fine products by Mefites! Has anyone had luck with Buspar?

Speman testimonials 100
Buspirone is an anti-anxiety anxiolytic drug sold in the United States under the brand name of BuSpar. It is also available under its generic name.
2018 ©